
WHPP Key Insights on HPA Inspections
The Workplace Hazardous Materials Bureau recently hosted a WHPP Multi-Stakeholder Workshop on November 20, 2025. A highlight of the event was a comprehensive presentation by Katriona MacNeil, WHMIS Coordinator and HPA Inspector, providing valuable insights into the inspection process within Canada.
The presentation clarified the mandate of Hazardous Products Act (HPA) Inspectors and their principal roles in supporting Health Canada. These key personnel are responsible for monitoring compliance with the legislative requirements governing hazardous products in Canadian workplaces.
Inspections are initiated for various reasons and are instrumental in ensuring compliance with the HPA and the Hazardous Products Regulations (HPR). Organizations are identified for inspection based on several criteria.
The presentation detailed three main categories of inspections:
- Regular Inspection: Conducted proactively to monitor and verify ongoing compliance with the requirements stipulated under the HPA and HPR.
- Focused Inspection: Designed to assess a specific area of interest or concern regarding the requirements of the HPA and HPR.
- Compliance Verification Inspection: Initiated to verify compliance in response to information concerning known or suspected non-compliance with the applicable requirements of the HPA and the HPR
During an inspection, officials focus their review on documentation critical to WHMIS compliance, specifically as outlined under the HPR. This typically includes:
- WHMIS Labels
- Safety Data Sheets (SDSs)
- True copies of documents
- Sales or purchasing records
Inspectors may, in some cases, schedule the inspection in advance. During the visit, they typically request a review of the following, though the list is not exhaustive:
- Product Review: Onsite review of a selection of products (typically 2 to 5).
- Documentation Copies: True copies of the labels and SDSs for the hazardous products under review.
- Transaction Records: Purchase and/or sale information.
- Post-Visit Review: SDS and label information for a comprehensive post-visit review alongside other submitted documentation.
Crucial Requirement: Both English and French components of the SDS and label are required and may be assessed, as per the HPR stipulation for bilingual documentation in Canada.
The workshop also shed light on five of the most frequently observed non-compliances identified by inspectors.
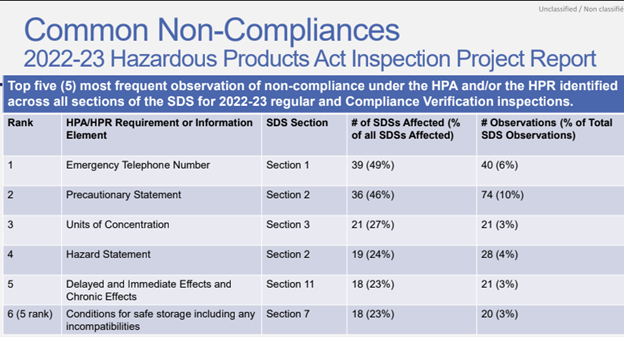
Copyright: November 2025, Katriona MacNeil, WHPP Multi-Stakeholder Workshop Hazardous Products Act (HPA)
It is essential that the SDSs utilized within your workplace are fully compliant with the current HPR regulations. With a looming deadline for compliance updates, ensuring your documentation is current is vital to avoid potential regulatory inspections from Health Canada inspectors.
If your organization requires assistance in authoring or updating Safety Data Sheets to meet Canadian/US legislative requirements, please contact us for a quote. We are committed to helping you achieve and maintain compliance with the legislation.
Ensure your team is fully aligned with current HPR requirements. Connect with ICC for SDS updates, WHMIS support, and expert guidance tailored to your compliance objectives.
Stay up to date and sign up for our newsletter!
We have all the products, services and training you need to ensure your staff is properly trained and informed.
 SDS Services SDS Services |
 OSHA / WHMIS / GHS OSHA / WHMIS / GHSTraining Courses |
 OSHA Publications OSHA Publications |



 ICC USA
ICC USA ICC Canada
ICC Canada